Absorption Experiment
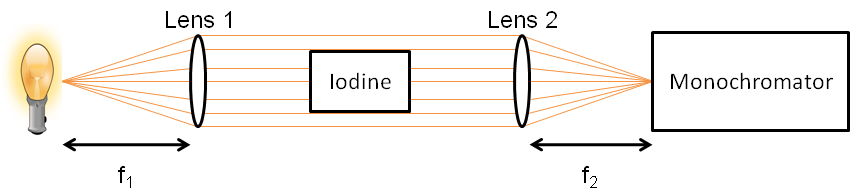
Components
- Light source: helium or mercury lamp.
- Lenses:
- The first lens collimates the beam (i.e., it makes all the rays parallel). The distance between the lens and the light source must be equal to the focal length of the lens, f1.
- The second lens focuses the light into the monochromator. Again, the distance between the lens and the monochromator must be equal to the focal length of the lens, f2.
The setup is arranged as seen in Figure 1. In order to understand the optics involved and learn more about focal length, watch this video.
Laser Induced Spectroscopy (LIF)
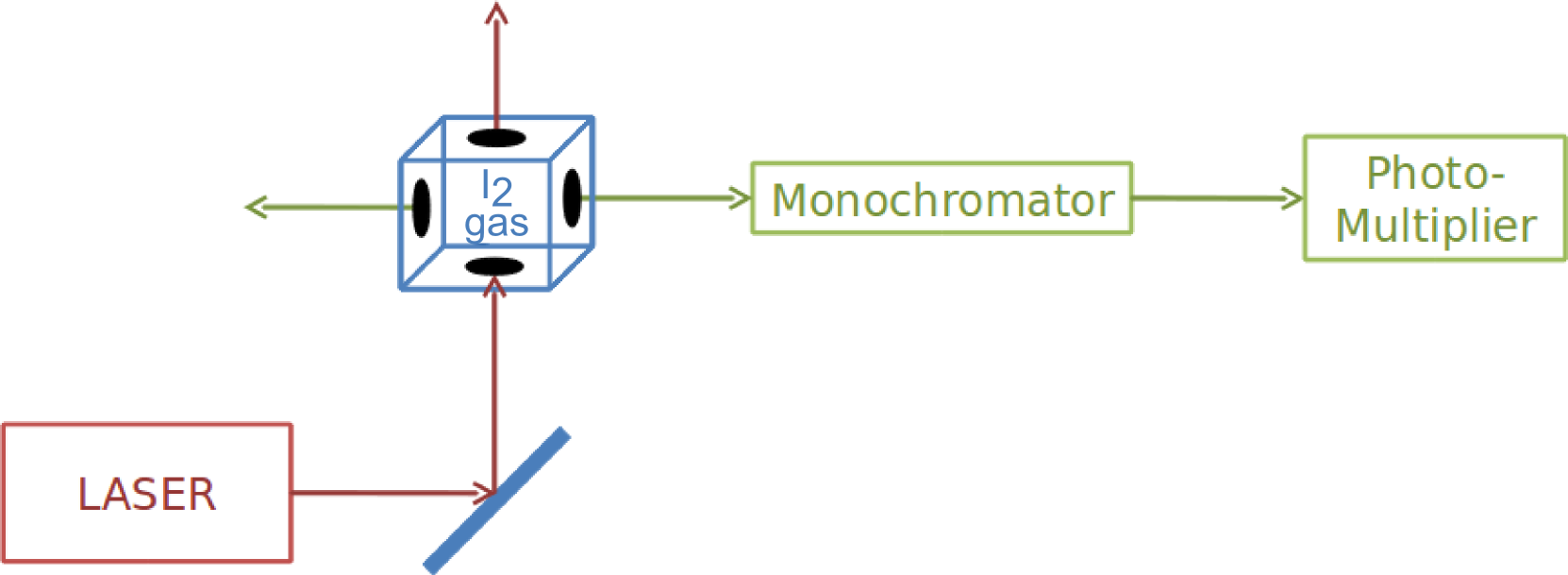
Components
- A He-Ne laser: This laser is inexpensive and simple to operate and is therefore the most common laboratory laser. It generates radiation with wavelength of 632.8nm. The laser is rather monochromatic (Δλ= 0.002nm). It allows us to resolve the rotational structure of the transition for the iodine gas.
- Mirror.
- Iodine chamber with holes at the side and bases: The laser light continues in a straight line in an upward direction, whereas the fluorescence is omni-directional and radiates through the side holes as well. This allows us to separate the laser light from the fluorescence light.
- Monochromator.
- Photomultiplier.