Surface Tension
In this experiment you will explore the concepts of surface free energy, adhesion and wetting
Preparation
- Answer the following questions in writing:
- What is the measurement of "contact angle" and is it useful for?
- What is the meaning of a high or low contact angle result, regarding a liquid and its interaction with the solid surface?
- Predict the trends you are expected to observe in every part of the experiment. Explain your predictions.
- How is a change in surface tension of a liquid-solid interface possible?
- Explain the terms of hydrophobicity and hydrophilicity. Use examples from daily life.
- In order to extract the surface tension on a teflon surface using the Zisman plot, you are required to plan the relevant concentrations (X) of n-propanol in water. Use the following equation to calculate γLG:
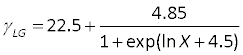
Remember that this equation is true only for 0.001 ≤ X ≤ 1, where X is the molar fraction of alcohol in water. - For comparison to the experimental values, find the following values in literature:
- Contact angles of water on aluminium, copper, PVC, teflon and glass.
- Surface tension, γSG, of teflon and air.
- The critical concentration of TX-100 (CMC) for maximal wetting.