Introduction to Planetary Science
Recommended reading: Worlds Apart by G. J.
Consolmagno and M. W. Schaefer. Prentice
Hall,
1.
Introduction to the introduction
1.1. What
do we see in the sky?
1.1.1. Clouds.
1.1.2. Meteors.
1.1.3. Comets.
1.1.4. Birds.
1.1.5. Sun.
1.1.6. Moon
1.1.7.
1.1.8. Stars
1.1.9. Planets
– Planets are different from stars as will be explained below.
1.2. What
are planets from the observational standpoint?
1.2.1. There
are fixed stars in the sky.
1.2.2. They
are arranged in constellations.
1.2.3. They
rise and set like the sun. Every night
the same stars rise a little earlier.
This was originally explained by saying that the stars sat on a celestial sphere that rotated about
the Earth.
1.2.4. There
are some “stars” that behave differently from the others. Whereas the ordinary stars are arranged in
constellations and their relative positions are fixed, there are “stars” that
wander through the sky. The Greek name
for these wanderers is “planets”.
1.2.5. The
planets are limited to a strip of the celestial sphere called the zodiac.
.
1.2.6. Planets
have a complex motion with respect to the background stars, which includes retrograde motion. The ancient
astronomers tried using epicycles to
explain this motion, but the number of epicycles needed for a good fit to
observations grew too large to be believable.
Now we explain it with a heliocentric
theory that has the Earth and all the planets going around the Sun. This theory is not only conceptually simpler,
but also gives a much better fit to observations.
1.2.7. The
heliocentric theory also explains the changes in the seasons.
1.2.7.1. Seasonal
changes involve not only changes in weather, but also changes in the stars
overhead.
1.2.7.2. The
Earth's rotation axis is tilted
23.5° from
the vertical to the ecliptic and
the direction of the axis is fixed in space.
1.2.7.3. When
the axis points towards the Sun, the northern hemisphere gets more sunlight and
it is summer there. Half a year later
the southern hemisphere experiences summer.
.
2.
What can we learn about this solar
system that we live in?
2.1. How
big is the solar system, i.e. what are the distances between the planets?
2.2. The
Greeks noted that Venus is never more than 47° from the Sun, and Mercury is never more than 28° from
the Sun.

2.3. At
maximum elongation the angle Earth-Planet-Sun = 90°.
2.4. Call
the distance from the Earth to the Sun 1 AU (astronomical unit) then
2.4.1. The
distance of Venus from the Sun is sin 47 = 0.7 AU.
2.4.2. The
distance of Mercury from the Sun is sin 28 = 0.4 AU. This assumes the orbits
are all in the same plane.
2.5. We
still need to know big an AU is.
2.5.1. In 275 B.C.E. Aristarchus of
2.5.2. Actually,
the correct angle is 89.85°
and the true factor is 390.
2.5.3. Since
the Sun was so far away, and Mercury and Venus orbited it, Aristarchus
suggested that the Earth orbited it as well (1700 years before Copernicus).
2.5.4. We
still don't have a distance in km. however.
The Greeks never solved the problem, but did solve others.
3.
How big is the Earth?
3.1. About
225 B.C.E. Erastothenes of

3.2. If
you assume that the Earth is flat this puts the sun at a height of 6400
km. . This is much too close. What is wrong?
3.3. The
angle is not caused by a nearby Sun, but by the fact that the Earth is
round. The Greeks surmised that this is
the case because
3.3.1. The
Moon is round.
3.3.2. The
Sun is round.
3.3.3. They
could see the Earth's shadow on the moon during a lunar eclipse.
3.3.4. The
tides are similar in the
3.3.5. He
even suggested you could sail westward from
3.4. So
you assume the Sun is much further away than an Earth radius so that its rays
are parallel. You also need to assume
that
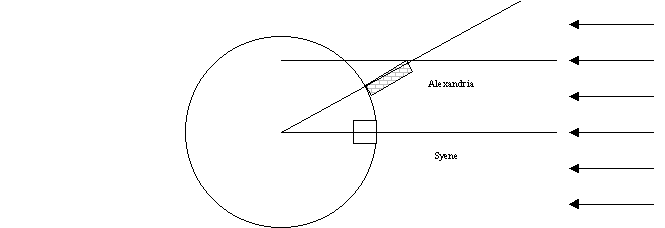
3.5. Geometry
then gives you that the radius of the Earth is 8x5000 = 40,000 stadia or about 6400 km. The circumference is then 40,200 km, which is
close to the correct value of 40,030 km.
3.6. If
we assume that the other planets are similar to the Earth, they too should be
thousands of km in diameter.
4.
Hipparchus tried to measure the A.U. in
150 B.C.E
4.1. When
the Moon eclipses the Sun, the two bodies appear to be nearly the same size.
4.2. When
the Moon eclipses the Sun, the Moon's shadow is very small, essentially a
point.
4.3. By
similar triangles, since the Sun is 19 times further than the Moon
(Aristarchus, 275 B.C.E.), the Sun must be 19 times larger than the Moon, but
how large is the Moon?
4.4. When
the Earth throws its shadow on the Moon, that shadow is much larger than the
Moon (2.5 times). Since we know the
diameter of the Earth, we can set up a system of equations based on similar
triangles: Let EM be the Earth-Moon distance, M be the diameter of the Moon, D
the diameter of the Earth, and x the distance from the apex of the Earth's
shadow to the Earth. We then have, from
the big triangle

![]()
4.5. The
little triangle gives
![]()
4.6. The
first equation gives
![]()
4.7. while
the second gives
![]()
4.8. Equating these two relations gives M = 0.30D. We now have a value for the diameter of the Moon: 3800 km. The actual value is 3450 km. We have learned that the Moon is a planetary-sized body.
4.9. From Aristarchus we know that the Sun is 19 times larger than the Moon, and has a diameter of 73,000 km. In fact the solar diameter is about 20 times larger, but that is due to the error Aristarchus made in measuring the angle.
4.10. The
distances can be found by measuring the angle distended by the Moon. The result gives a distance EM = 450,000 km
(the real value is about 400,000 km), and the distance to the Sun as 7,600,000
km (again about a factor of 20 wrong).
In any case, we have some notion of the size and distance of solar
system objects.
5.
A much more accurate way of measuring
the distance to the Sun is to use transits of Mercury and Venus.
5.1. The
angular size of the Sun is about 0.5°, so the diameter of the Sun is sin 0.5 = 0.01 AU.
5.2. We
can measure the diameter of the Sun by observing a transit of Mercury or Venus.
5.3. If
you observe the time to transit from two places on Earth, the parallax allows you to compute the Sun's
diameter in terms of Venus' distance from the Sun, and hence in terms of the
AU. This lets you find the AU directly.
5.4. Because
of the tilt of the orbit, transits are rare.
Kepler (1629) found that, for Venus, they occured in pairs, 8 years
apart, about once a century.
5.4.1. Jeremiah
Horrox observed a transit in 1639, and realized how it might be used, but never
published details.
5.4.2. James
Gregory elaborated on the idea in 1663.
5.4.3. Edmund
Halley (1716) gave all the details. The
next transits were in 1761 and 1769, and Halley left detailed instructions on
how to do the calculations. The story of
these observations is fascinating.
5.4.4. The
value was improved during the transits of 1874 and 1882, and they give the
value of 1AU = 150,000,000 km that is used today.
5.4.5. The
next transits will occur on
6.
Other sizes and distances.
6.1. The angular diameter of Venus is about 1 arc-minute. From this and the AU we get the diameter of Venus to be about 12,000 km, just less than that of the Earth.
6.2. Other
planetary distances and diameters can be found from Kepler's laws and similar
considerations.
7.
The Sun
7.1. What
is the Sun? We all know it is a star,
but what about it's other properties.
7.2. We
can measure the radius of the Sun. Rs
= 6.96x1010 cm = 109 Re.
7.3. We
can measure the mass by using the size of the AU and the fact that the Earth
orbits in one year. Equating the
centrifugal and gravitational forces

where
v is the speed of the Earth in its
orbit around the Sun. The can be found
from

where
T is the time it takes the Earth to
make one orbit. The result is Ms
= 1.99x1033 g = 333,000 Mearth.
7.4. The
Sun is orders of magnitude larger than the Earth.
7.5. We
are 1.5x1013 cm from the Sun, but we still feel its warmth.
7.6. The
energy flux through any sphere concentric with the Sun must be constant if
there are no other sources or sinks, so if L is the rate of energy production
(erg s-1) and R is the distance from the sun, then
![]()
7.7. The
luminosity is also related to the effective temperature by
![]()
7.8. From
this it follows that if Rs and Ts are the radius and
temperature of the Sun, and re and Te the distance from
the Earth to the sun and its temperature, then the amount of energy put out by
the Sun per second is
![]()
7.9. and
the amount of energy reaching the Earth per square centimeter times its cross
section is

where re
the radius of the Earth. We can equate
this with the energy reradiated by the Earth in steady state (4p s Re2
Te4).If we take Te = 280 K, we find that Ts
= 5800 K. The Sun is hot.
7.10. We
can also compute the luminosity. L =
3.9x1033 erg s-1.
So there is an enormous energy source inside the Sun.
7.11. Can
we check any of this?
7.11.1. We
can estimate the surface temperature from a black body.
7.11.2. A
body in equilibrium with a surrounding electromagnetic field (like in a heated
cavity) will give the radiation a form that is related to its temperature. The energy density at a given wavelength, l, is given by

7.11.3. where
k is Boltzmann's constant c is the speed of light, and h is Planck's
constant. This is known as the Planck function. If we differentiate with respect to
wavelength, and substitute for the various constants we get the Wien displacement law lmaxT = 0.3 cm K.
7.11.4. If
we plot the amount of energy the Sun emits at different wavelengths, we can get
something that looks like a black body curve. It won't be exactly right because
7.11.4.1. The
light comes from different layers at different temperatures.
7.11.4.2. Some
of the light gets absorbed on its way out.
7.11.5. In
reality you need detailed models of the radiative transfer, but what
comes out is that the temperature really is around 6000 K near the surface.
7.12. The
Sun contains most of the mass (99.9%) of the solar system, and therefore the
center of mass of the solar system is close to the center of mass of the Sun.
8.
What is the Sun made of?
8.1. The
mass and radius give us a density of 1.41 g cm-3.
8.2. Does
that mean that the Sun is composed of water + some heavier material, or is this
just an effect of pressure. What is the
pressure inside the Sun?
8.3. Imagine
a spherical shell inside the Sun with an inner radius of r and a thickness
dr. The pressure at the inner surface is
P(r) and at the outer surface P - dP.
The difference, dP comes from the additional weight of the layer itself. The layer has a volume 4p r2
dr. If the density in this region is r(r) then the
weight is the density times the volume, and the pressure is the weight divided
by the area. Thus
![]()
![]()
8.4. This
is the equation of hydrostatic
equilibrium. In deriving it we have
assumed that the material making up the body has no internal forces (i.e. it
acts like a liquid ... the hydro in
hydrostatic). We have also assumed that
the configuration is not accelerating (... static). Finally, for simplicity in deriving the
equations I have assumed that the body is a sphere, and that the density
distribution is spherically symmetric, but these assumptions are not essential
to the argument.
8.5. There
are still more unknowns than equations, but we can easily find a relation
between M(r) and r(r) by
demanding mass conservation:
![]()
![]()
8.6. We
still need some relation between the pressure and the density (equation of state), but this depends on
the composition, and the temperature.
Thus we need to introduce another variable, T(r). The variation of T will depend on details of
energy transfer, so we need to include information about opacity, conductivity
and convection. We will look at some of
these issues later. For the moment we
ignore these details.
8.7. The
pressure equation gives

8.8. We
can define an average density, <r(r)> as the average density of the material contained in
the volume within a distance r from the center:
![]()
so that

which can be
substituted to give

8.9. We
make two assumptions
8.9.1. P(R)<<P(0)
8.9.2. r(r) does not
increase as r increases. This means that
<r(r)> ³ <r(R)>
8.10. This
gives

8.11. On
integration we get
![]()
which gives an
lower limit to the central pressure. For
the case of the Sun, this corresponds to a pressure of P(0) ³ 103
Mbar. In fact, since the central
densities are much much higher than the average density, the central pressure
is expected to be much higher than this as well. For the Earth, where the
density changes are much less, this approximation gives a central pressure of
about 1.5 Mbar, while detailed models give a pressure of 3.5 Mbar. In any case, the pressure in the Sun is
enormous.
8.12. The
temperature inside the Sun can be estimated.
8.13. The
gravitational binding energy of the Sun is roughly
![]()
8.14. The
thermal energy (assuming the Sun has some characteristic temperature) is
![]()
where N0
is Avogadro's number, k is Boltzmann's constant, and m is the mean
molecular weight. Taking m = 1 as
representative, and equating the thermal and gravitational energies, we get T ~ 107
K which is the right order of magnitude.
8.15. Assuming
an ideal gas, we have
![]()
which gives a
density of the order of 1 g cm-3.
This is consistent with the assumption that the density is everywhere
roughly equal to the average density.
Since it is not, and the pressure at the center is really much higher,
the actual density there will be much higher.
8.16. We
can now begin to understand how the Sun gets its energy ... from nuclear
reactions.
9.
The Sun like all other stars has a
nuclear reactor in its center, and that supplies the energy. This is the difference between stars and
planets.
9.1. The
large mass of the star produces huge pressures and temperatures near the
center.
9.2. If
the configuration is to remain stable against collapse, it must be very
hot. The thermal energy is supplied by
nuclear reactions.
9.3. A
neutron, left to itself is unstable and breaks up into a proton, electron, and
anti-neutrino. The reverse reaction p ® n + e+ + n does not
occur naturally since it violates conservation of energy. In the presence of a second particle, some
energy can be “borrowed” from the surrounding field, and the reaction can
proceed. This requires high density and
temperatures of about 107 K.
It is also rare.
9.4. If
it does happen and the resultant neutron combines with a proton, then resulting
reaction is p + p ® D + e+
+n. The cross section is 10-47 cm2
under the appropriate conditions and it produces 1.28 MeV of energy.
9.5. The
resultant D will immediately combine with a nearby proton to give D + p ® 3He
+ g. I will write this as D(p,g)3He. The reaction releases 5.49 MeV.
9.6. The
3He could wait around until another proton turned into a neutron in
its presence, and then capture it to become 4He, but that is
extremely rare. Instead, 3He
builds up until one helium can give up its neutron to another to make 4He. The reaction is written as 3He + 3He
® 4He
+ 2p and releases 12.9 MeV of energy. No
nuclei of atomic number 5 exist, so the sequence terminates here. This is the sequence of reactions that keeps
the Sun burning.
9.7. About
10% of the Sun is under the right conditions for this to happen.
10. How
does this energy get out of the Sun?
10.1. The
resultant energy is produced as gamma ray photons. These are not absorbed in the Sun since there
is nothing to absorb them, but they do get scattered. They have a random walk with a mean free path
that is very short and it takes millions of years for them to reach the outer
layers of the Sun. Even so radiation is
the most efficient method of transferring energy. For the Sun the mean free path of a photon is
roughly a cm. The radius of the Sun is
about 7 x 1010 cm, and a random walk with n steps of size x will
carry you approximately a distance of xÖn, so to travel through most of
the Sun, the photon will have to take 1022 steps, and so travel a
total distance of »1022
cm. Even at the speed of light, this
takes some 104 years.
10.2. In
the outer layers the temperature is low enough so that electrons and nuclei can
recombine. At this point a gamma ray can
tear them apart again and be absorbed in the process. Since the heat cannot be transferred
efficiently by radiation the temperature gradient builds up until convection
begins.
10.2.1. Convection
is a much more efficient process process for transferring energy than
radiation. The density difference
between hot matter and colder matter is
![]()
and the upward
buoyant force per unit volume is
![]()
which gives an
acceleration of
![]()
10.3. The
time to travel the radius of the Sun, taking DT/T = 1, M = 2
x 1033 g, and r = 7 x 1010 cm, is
 sec.
sec.
10.4. This
is much much faster.
10.4.1. The
problem is that it only happens under special conditions. Consider a blob of matter rising to a region
of lower temperature. Since the pressure
is lower there, it also expands and cools adiabatically. It will rise from a region where the pressure
is P, the temperature, T, and the density r, to a region where the pressure is P',
etc. The blob will continue to rise so
long as it is less dense than its surroundings, i.e. rblob < r'. Since the pressure inside the blob is equal
to that outside, this means that in order for convection to be active we need
T' < Tblob. But T' = T -
dT, while Tblob = T - dTadiab, so the condition for
convection is
![]()
so that
convection will only start up in a region where the temperature gradient is high
enough, i.e. where the opacity is high enough.
10.5. The
Sun is thus built up of a radiating core, a convecting layer near the surface,
and then an outer radiating region called the photosphere.
. Some
of the convection reaches the surface, and we see granulation.
10.6. Above
the photosphere is the chromosphere,
a thin atmosphere of gas extending about 2000 km. .
10.7. Above
that is the corona. . This region can actually reach
temperatures of 106 K, which would seem to violate the first law of
thermodynamics. In fact it is heated
from below by mechanical means, and has an unstable outer layer where the solar
wind is formed
11. Sunspots
11.1. Sunspots
can be seen easily on the Sun.
11.2. The
background temperature in the Sun is about 5770 K, while in a sunspot it is
about 4500 K. As a result they must be
denser than their surroundings, and so must somehow be supported against
gravity.
11.3. They
are found between 5° and 40° from the
equator, and are seen in pairs.
11.4. They
move slowly across the surface of the Sun, and the number changes with
time. There is an 11-year cycle, which
culminates in a time when there is a minimum of sunspots. This is called the quiet Sun period
11.5. The
explanation of this phenomenon is that the sunspots are regions where the Sun's
magnetic field is breaking through the surface.
The background field is typically 2 x 10-4 telsa, but in
sunspots it can be 0.0025 to 0.05 telsa.
Sunspots appear in pairs, one being where the field emerges at the
surface, the other where it re-enters the Sun.
At the end of the 11-year cycle the field changes polarity, so that a
complete cycle is 22 years (see picture of sunspot pair).
11.6. Magnetic activity in the Sun is also responsible
for solar flares and for the solar wind.
(see picture of solar flare)
12. Solar
neutrinos
12.1. It
would be very nice to be able to check our theories by looking into the center
of the Sun. This is possible through neutrino
astronomy. Neutrinos have a very small
cross-section for interaction with matter, so you fill a gold mine (to keep out
extraneous radiation) with 400,000 liters of C2Cl4, and
look for the reaction
37Cl + n ® 37Ar
+ e-
which should produce
a few atoms of radioactive argon per month.
You flush out the detector ever few months and count the number of
atoms. At present the experimental value
is 2.1 ± 0.3 SNU
(Solar Neutrino Units) while the best theoretical models give 5.8 ± 2.2 SNU. The agreement is not very good and people have
been looking for possible explanations.
12.2. One
problem is that this experiment sees only high-energy neutrinos. The sequence
p + p ® D + e-
+ n
D + p ® 3He
+ g
3He + 3He
® 4He
+ 2p
produces neutrinos with a maximum energy of 0.42 MeV. These are not energetic enough to be detected by the chlorine experiment. Instead we see the neutrinos from a less common reaction sequence
3He + 4He
® 7Be
7Be + p ® 8B
+ g
8B ® 8Be*
+ e+ + n
8Be* ® 24He
12.3. This
reaction produces 14 MeV neutrinos and these are seen. It would be nice to check the first reaction,
which is far more important. These less
energetic neutrinos can be detected via the reaction
71Ga + n ® 71Ge
+ e-
but for this
you need about 30 tons of Ga, which is a rare earth. Currently the GALLEX detector is being run
using GaCl3. We should know
more in the next few years.
12.4. A
second possibility comes from high-energy physics theories that suggest that
the neutrino has a finite rest mass. In
this case some neutrinos might switch to another type on their way to Earth,
and thus escape detection.
12.5. The
internal structure of the Sun can also be probed by heliseismology. Here you study the vibrations of the Sun in
an attempt to determine the density distribution inside it. This is a relatively new field, but the
results tend to confirm the picture developed from other means.
13. Nucleosynthesis
13.1. Where
do the elements that make up our solar system come from, and what are their
abundances?
13.2. H
and He were formed mostly in the big bang, although some He is formed in stars.
13.3. We
have seen how hydrogen burns to form helium, but this is a slow reaction. There is an alternative that is important is
some stars
p + 12C
® 13N
+ g
13N ® 13C
+ e+ + n
13C + p ® 14N
+ g
14N + p ® 15O
+ g
15O ® 15N
+ e+ + n
15N + p ® 4He
+ 12C
or
15N + p ® 16O
+ g
16O +p ® 17F
+ g
17F ® 17O
+ e+ + n
17O + p ® 4He
+ 14N
13.4. This
is known as the CNO bi-cycle, and can only work in stars that have a background
supply of 12C to begin with, i.e. stars that are made of material
from an earlier generation of nucleosynthesis.
13.5. When
the supply of hydrogen in the core of the star is exhausted it collapses under
the pressure, and will continue to collapse unless another fuel is found. 4He + 4He ® 8Be
won't work because it is unstable and decays back to He in 10-16
s. But it can hold the two heliums
together long enough for a third He to join and form 12C. This happens under conditions of T = 108
K and very high density, i.e. after the core has contracted. In such a case the outer envelope of the star
expands and we get a red giant.
13.6. When
this fuel is exhausted, C burning begins and helium is added to form 16O
or two 12C's are combined to give 24Mg. 20Ne can be formed in this way and
so can all the elements up to 56Fe, but only if their atomic number
is a multiple of 4. As long as the star
is massive enough it will continue to contract until the next burning stage is
reached. If it is not massive enough,
the contraction is halted when the material cannot be squeezed any
further. The result is a white dwarf, followed by a red then black dwarf.
13.7. If
the star is massive enough it can get all the way to 56Fe. At this
stage something else happens. 56Fe
is the most tightly bound nucleus.
Further fusion requires addition of energy. Fission does too, so the ensuing collapse is
not halted and becomes catastrophic. The
result is a type II supernova. This is
an excellent means of mixing the elements made till now in with the rest of the
interstellar medium.
13.8. What
about the rest of the elements that are not multiples of 4?
13.9. There
are still protons around, so we can get
p + 20Ne
® 21Na
but this is
unstable and decays into
21Na ® 21Ne
+ e+ + n
so we have produced
a nucleus with atomic number 21. But
this can react with a He to give
21Ne + 4He
® 24Mg
+ n
13.10. The
magnesium we could have gotten from previous reactions, but here the
interesting thing is the neutron. It can
combine with some other nucleus (like 66Zn) to form a heavier
isotope, and to follow a path known as the S-process (slow process).
13.11. If
there are reactions that produce many neutrons, the reaction path will follow
that of the R-process (rapid
process). Other processes occur as
well. These happen in various stages of
stellar evolution such as novae and supernovae.
The point is that current elemental abundances depend on the history of
nucleosynthesis in the galaxy.
14. So
how is a star different from a planet?
14.1. Perhaps
the first question to ask is how planets differ from ordinary rocks. The thing that keeps me from going through
the floor is the repulsion between atoms and molecules. These sit in lattice sites in the solid, and
what makes the material solid is the forces that keep the molecules in those
sites. We can think of these as springs
holding the particles in place. If I
increase the gravitational force (i.e. the pressure on the material) I can
affect those springs. Typically, the
pressures involved are about 106 - 108 dynes cm-2. We can make a simple estimate of how large a
body has to be to get these pressures.
The pressure is the force divided by the area. The area is approximately 4p R2,
and the force is the force of gravity, Mg.
![]()
![]()
![]()
14.2. For
a planet with a density of about 1 g cm-3 (which is typical for an
icy body), this gives R » 100 km. Icy bodies
larger than this would be expected to be round.
Rock is stronger than ice, but its density is higher as well, so the
numbers stay about the same.
14.3. As
the pressure increases, the material flows, but the atoms and molecules still
maintain their identities. Eventually
the molecules break down, and shortly thereafter the atoms do too. At this stage we may say we have gone from
planet to star.
14.4. In
a simple (e.g. Bohr) model of an atomb
the nucleus has a radius of about 10-13 cm, and the electron cloud
has a radius of about 10-8 cm.
For a hydrogen atom, the energy of the electron in the field of the
proton is
![]()
where p is the
momentum, e the electric charge, m the mass of the electron, and r the distance
from the nucleus. The quantum mechanical
wavelength of the electron is l =h/p where h is Planck's constant. If R is the radius of the electron's orbit,
and l = 2p R, then we
get
![]()
for stability
we want this energy to be a minimum, i.e. dE/dR = 0. This gives
![]()
14.5. a0
is called the Bohr radius, and is a
natural unit of atomic distance. The
corresponding energy is
![]()
14.6. This
unit of energy is called a Rydberg
and is the natural unit of atomic energy.
For the case of an atom with Z electrons, az = a0/Z
and Ez » ZE0. The equality is not exact because of
shielding from the inner electrons.
14.7. The
pressure associated with the electron cloud can be estimated from
![]()
14.8. E
is composed of a kinetic and a potential term, so P can be written as P = Pk
+ Pv where (for a hydrogen atom)
![]()
which
represents the repulsion due to the kinetic energy of the electron which keeps
it from falling into the nucleus, and
![]()
which is the coulomb attraction.
14.9. At
R = a0 the pressures are equal and the atom is in equilibrium. At this point the pressure is about 300
Mbar. This is roughly the pressure at
which atoms begin to break down, and may be considered to define the place
where the body stops being a planet.
This occurs at a radius of around 1010 cm. The radius of Jupiter is around 7 x 109
cm, so it is near the upper limit of what we would consider a planet.
14.10. A
star also burns, and more detailed calculations show that this begins at masses
of about 70 Jupiter masses. The range in
between is filled with brown dwarfs. These are objects that are hot because of
their accretional energy, but are not massive enough to be stars. Because they are hot, they glow for a while
(some 109 - 1010 years depending on mass), hence the
name.
They
can be identified by their mass if they are in orbit, and by the presence of Li
in the atmosphere if they are isolated (Li is quickly burned in any nuclear
reactions). Some failed stars (those that burn deuterium only) will also burn
Li, so there is some ambiguity. But a number of brown
dwarfs have been found and it has become a new field of study.