Definition
Neoplasia is new, uncontrolled growth; a "tumor" or "mass lesion" is simply a "growth" or "enlargement" which may not be neoplastic (such as a granuloma). The term "cancer" implies malignancy, but neoplasms can be subclassified as either benign or malignant.
Benign Neoplasms
Characteristics include slow growth, resemblance to tissue of origin (well differentiated), circumscription, lack of invasion or metastases.
Usually solitary (e.g., lipoma of colon, meningioma of dura), but may be
multiple (e.g., leiomyomata of uterus, intradermal nevi on skin). May cause
problems through mass effect, particularly in tight quarters (pituitary adenoma
in sella turcica).
A hamartoma is a peculiar benign neoplasm which is a localized but haphazard growth of tissues normally found at a given site (pulmonary hamartoma has jumbled cartilage, bronchial epithelium, and connective tissue)
A choristoma is a benign neoplasm consisting of tissue that is not normal to
the site of origin (e.g., salivary gland choristoma of the middle ear).
Malignant Neoplasms
Characteristics include more rapid increase in size, lack of differentiation (anaplasia), and tendency to spread by invasion or metastases.
Cytologic features of malignant neoplasms include:
- increased nuclear size (with increased nuclear/cytoplasmic ratio--N/C
ratio).
- variation in nuclear or cell size (pleomorphism).
- lack of differentiation (anaplasia).
- increased nuclear DNA content with subsequent dark staining on H and E
slides (hyperchromatism).
- prominent nucleoli within the nuclei.
- mitoses (especially irregular or bizarre mitoses).
All of these features are "atypical". Atypia implies a change for the worse from normal.
Spread of Malignant Neoplasms
- By direct extension (invasion) into surrounding tissues.
- Through lymph channels to lymph nodes (lymphatic spread)--typical of
carcinomas.
- Via the bloodstream (hematogenous spread)--typical of carcinomas or
sarcomas.
- Within body cavities (seeding)--typical of neoplasms in peritoneal cavity.
The spread of malignant neoplasms determines the stage
- In-situ (refers to epithelial malignancies confined to just the epithelium
without going throug the basement membrane).
- Microinvasion (spread of epithelial malignancies just beyond the point of
origin through the basement membrane).

- Local invasion (spread within the organ of origin or to contiguous
structures).
- Local metastases (non-contiguous spread of the neoplasm within the organ
of origin or to the lymph nodes closest to the organ of origin).
- Distant metastases (spread to other organs or to far away lymph nodes)
The indicators that a neoplasm is malignant are:
- Metastases (best indicator) and
- Invasion (next best indicator)
Grading of neoplasms
Based upon: differentiation (the degree to which the neoplasm resembles the tissue of origin) along with the degree of atypia.
- well-differentiated--looks very similar to normal tissue
- moderately differentiated--looks something like normal
- poorly differentiated--hardly looks like normal tissue
- anaplastic--virtually no similarity to normal tissue
Both staging and grading schemes are devised to classify malignant neoplasms for determining appropriate treatment and to try and determine the prognosis. In general, the higher the stage or the higher the grade, the worse the prognosis.
Appearances of Neoplasms
Desmoplasia refers to the proliferation of non-neoplastic connective tissue in association with neoplasia (gives tumors a firm, fibrous, "scirrhous"
appearance) and may distort surrounding tissues.
Neoplasms may mimic the tissue of origin, but not always. Larger masses
tend to undergo central necrosis. Metastases usually look similar to the
primary, but not always.
The primary site is ususally a single large mass in an organ, while multiple masses in an organ usually indicate metastases.
It is not always possible to tell benign from malignant based upon
histologic or cytologic criteria alone. The biologic behavior of a neoplasm may
not always correlate with the appearance, making choice of treatment more
difficult.
Oncogenesis
The process of neoplasia is represented symbolically below:

Nomenclature of Neoplasia
Based upon origin:
- Maligant neoplasms arising from tissue embryologically derived from
ectoderm or endoderm are usually carcinomas. Examples include:
- Squamous cell carcinoma of cervix
- Adenocarcinoma of stomach
- Hepatocellular carcinoma
- Renal cell carcinoma
- Malignancies arising from mesoderm are usually sarcomas. Examples include:
- Leiomyosarcoma
- Chondrosarcoma
- Osteosarcoma
- Liposarcoma
- Neoplasms with more than one cell type but arising from only one germ
layer are called "mixed tumors". The best example is the benign mixed tumor (also called pleomorphic adenoma) of salivary gland.
- Neoplasms with more than one cell type and arising from more than one germ
layer are called teratomas. Such neoplasms are common in the ovary.
- Neoplasms ending in "-blastoma" resemble primitive embryonic tissues. Examples include:
- Retinoblastoma
- Neuroblastoma
- Hepatoblastoma
- Medulloblastoma
- Not all malignant neoplasms have benign counterparts:
- Hematopoietic and lymphoid cells (as in bone marrow and lymph node) give
rise to leukemias and lymphomas. They have no benign counterpart.
- Gliomas (astrocytomas, oligodengrogliomas, glioblastoma multiforme, etc)
arise from glial cells in the CNS. They have no benign counterpart.
Carcinomas
Arise from epithelial surfaces (in gastrointestinal tract, in respiratory tract, in urogenital tract, in biliary tract, in skin) and in organs with epithelial-lined ducts (breast, pancreas, salivary gland, liver). Endocrine
glands, including testis and ovary, may also give rise to carcinomas. In
general, carcinomas are composed of polygonal-shaped cells.
- Carcinomas that form glandular configurations are called adenocarcinomas.
- Carcinomas that form solid nests of cells with distinct borders,
intercellular bridges, and pink keratinized cytoplasm are called squamous cell
carcinomas.
Sarcomas
Arise from soft tissues (connective tissues such as cartilage, bone, or fascia, smooth or skeletal muscle, blood vessels, lymph vessels, coverings of
organs such as mesothelium). In general, sarcomas are composed of very
pleomorphic spindle-shaped cells. Sarcomas are generally big and bad.
Biology of Neoplasia
This subject attempts to determine what gives rise to neoplasms and what controls their growth.
Causes of Neoplasia
The origin for many neoplasms is obscure. However, there are several
theories of origin:
Environmental causes:
- Chemicals that are man-made (such as aniline dyes and bladder cancer),
drugs (cigarette smoke and lung cancer), and natural compounds (aflatoxins and
liver cancer) which are carcinogenic.
- Oncogenic viruses such as human papillomavirus (HPV) implicated in most
squamous cell carcinomas of cervix and anogenital squamous papillomas,
Epstein-Barr virus (EBV) implicated in African Burkitt's lymphoma, and hepatitis
B virus (HBV) implicated in development of hepatocellular carcinomas.
- Radiation (such as ultraviolet light and skin cancers; gamma radiation and
leukemia, thyroid, lung, colon, and breast cancers). Ultraviolet light induces pyrimidine dimers in DNA. Ionizing radiation induces mutations in DNA.
Hereditary causes:
- Chromosomes which have absent or defective anti-oncogenes that control
growth (retinoblastoma results from defective chromosome 13)
- Obscure defects: racial predilections (American women have breast cancer
more often than Japanese women; Japanese men have stomach cancer far more often
than American men).
Age: older persons have a greater propensity to develop neoplasms from lack of effective control mechanisms.
Altered DNA:
- All of the above are probably mediated by the cause, whatever it is,
producing a mutation in, or damage to, cell DNA
- There can be mutations involving tumor suppressor genes (such as p53), which then fail to exert a controlling influence upon growth activation. The majority of human neoplasms probably arise via this mechanism.
- In some cases these mutations are probably mediated by proto-oncogenes (genes which control cellular growth) that undergo mutation to oncogenes which give rise to neoplasia. Proto-oncogenes can be activated by point mutations, translocations, and by gene amplification.
- An example of this is chronic myelogenous leukemia (CML) which is a neoplastic proliferation of white blood cells. All cases of CML have the "Philadelphia chromosome" which is a translocation between chromosomes 9 and 22. This translocation juxtaposes the proto-oncogene ABL with the breakpoint cluster region (BCR) on chromosome 22. The chimeric ABL-BCR gene leads to production of a mutant protein with enhanced tyrosine kinase activity. This protein may play a role in regulation of cell growth in CML.
- About 15 to 20% of human cancers have been linked to oncogenic activity. The ras oncogene is the transforming gene found most frequently in human cancers.
- Oncogenic viruses may bring oncogenes with them, so-called viral oncogenes
(typical of RNA containing "retroviruses" such as human T-lymphotropic
viruses (HTLV's).
- Growth factors such as epidermal growth factor (EGF), platelet-derived
growth factor (PDGF) and colony-stimulating factor-1 (CSF-1) assist oncogene
activity. Transforming growth factor (TGF-alpha) also promotes tumor growth.
Tissue evidence of carcinogenic factors at work
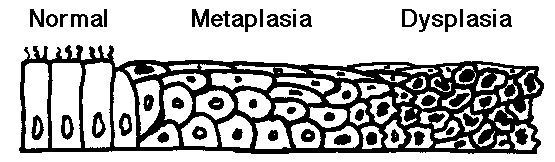
- Metaplasia: an initial change from normal cells to a different cell type
(such as chronic irritation of cigarette smoke causing ciliated pseudostratified
epithelium to be replaced by squamous epithelium more able to withstand the
insult).
- Dysplasia: an increasing degree of disordered growth or maturation of the
tissue (often thought to precede neoplasia) such as cervical dysplasia as a
result of human papillomavirus infection. Dysplasia is still a reversible process. However, once the transformation to neoplasia has been made, the process is not reversible.
- Thus, there is a natural history from metaplasia to dysplasia to
neoplasia. This is best evidenced in development of uterine cervix and
respiratory tract neoplasms.
Chemical carcinogenesis
There are two steps: initiation and promotion
An initiating carcinogenic agent irreversibly damages cell DNA (it is
mutagenic) to start the process. Examples of carcinogenic initiators include:
alkylating agents like cyclophosphamide, polycyclic aromatic hydrocarbons like
epoxides found in smoked foods, aromatic amines or azo dyes used in food
coloring, aflatoxins in moldy peanuts, nitrosamines in pickled foods.
A promoting agent (which may be the same as the carcinogen) then acts
(reversibly) to cause proliferation of a neoplastic cell clone, but there
appears to be a "dose-threshold" concentration of promoter below which
neoplasia will not occur. Examples of promoters include: hormones such as
estrogen, drugs such as diethylstibesterol, and chemicals such as cyclamates
used as sweeteners.
Cellular Transformation
- Some factor, as discussed above, causes a cell to be transformed to a
neoplastic cell that is not controlled by normal body processes. Probably most
transformed cells die because they are too abnormal to function or are abnormal
enough for the body's immune system to destroy them. However, if the factors
promoting neoplasia persist, a transformed cell may some day give rise to a
clone that does continue to grow.

- Malignant neoplasms do not tend to arise from benign neoplasms (e.g.,
malignant melanomas do not come from benign nevi) though in some cases such as
adenomas of the colon, the appearance of the benign neoplasm is a step toward
possible malignancy.
- There are "pre-cancerous" conditions in which malignant
neoplasia is more likely to occur (but not in every case): liver cirrhosis,
chronic ulcerative colitis, atrophic gastritis, epidermal actinic keratosis,
oral leukoplakia.
Clonality
- Neoplastic cells tend to be monoclonal, or similar in genetic makeup,
indicating origin from a transformed cell. Non-neoplastic proliferations (such
as reactions to inflammation) have cells that are polyclonal in origin.
- The concept of "tumor progression" holds that subclones may arise
over time from the original malignant clone. These subclones may differ from
the original clone in characteristics such as invasiveness, metastatic
potential, and response to therapy.
Tumor Genetics
Neoplasms have a greater tendency to karyotypic abnormalities such as
translocations, deletions, and gene amplifications (which are also activators of
proto-oncogenes). Leukemias and lymphomas are famous for this, as with the
Philadelphia (Ph1) chromosome of chronic myelogenous leukemia and the t(8:14)
translocation in Burkitt's lymphomas.
Tumor growth
- In general, the less differentiated a neoplasm, the faster it grows. The
cell cycle of neoplastic cells is not shortened, rather the growth fraction of
cells proliferating is increased. This is offset by neoplastic cell death.
Tumor growth is expressed as a "doubling time" or the time to increase
twice in volume (e.g., from 1 to 1.3 cm diameter). An aggressive malignant
neoplasm doubles in 1 to 3 months, while benign neoplasms double in years.
- Some neoplastic growth is influenced by host factors. Estrogenic hormones
aid growth of breast fibroadenomas or carcinomas and uterine leiomyomas because
the tumor cells have hormone receptors.
- Growth is also dependent upon the ability of the tumor to develop a blood
supply. Factors secreted by neoplastic cells promote angiogenesis and
fibroblast proliferation.
Characteristics of Transformed (Neoplastic) Cells
- Neoplastic cell growth is not inhibited by contact with surrounding cells
and is not dependent on anchorage to a solid surface.
- Neoplastic cells may attain "immortality" or the ability to keep dividing indefinitely.
- They are discohesive and transplantable--favoring invasion and metastasis.
- Tumor cells can bind to laminin and fibronectin in connective tissues,
then secrete collagenases or proteases, and then invade.
Epidemiology of Neoplasia
Neoplasms can be characterized by:
- Their incidence (how often they occur).
- Their death rate (how many deaths are caused by them).
A neoplasm such as basal cell carcinoma of the skin can be quite common, yet it almost never kills the patient. On the other hand, gliomas of the brain are uncommon but virtually always kill the patient. One in 5 Americans dies of
cancer.
Incidence of Neoplasia
(estimated new cases for 1996 for malignant neoplasms in the U.S.)
| Men | Women |
| Prostate | 317,000 | Breast | 184,000
| | Lung | 112,000 | Colon-rectum | 82,000
| | Colon-rectum | 68,000 | Lung | 78,000
| | Bladder | 38,000 | Endometrium | 34,000
| | Lymphomas | 35,000 | Lymphomas | 26,000
|
Deaths from Neoplasia
(top death-causing malignant neoplasms in the U.S. estimated for 1996)
| Men | Women |
| Lung | 94,000 | Lung | 64,000
| | Prostate | 41,000 | Breast | 44,000
| | Colon-Rectum | 27,000 | Colon-Rectum | 28,000
| | Pancreas | 14,000 | Ovary | 15,000
| | Lymphomas | 13,000 | Pancreas | 14,000
|
|
 Return to the tutorial menu.
Return to the tutorial menu.

