


|
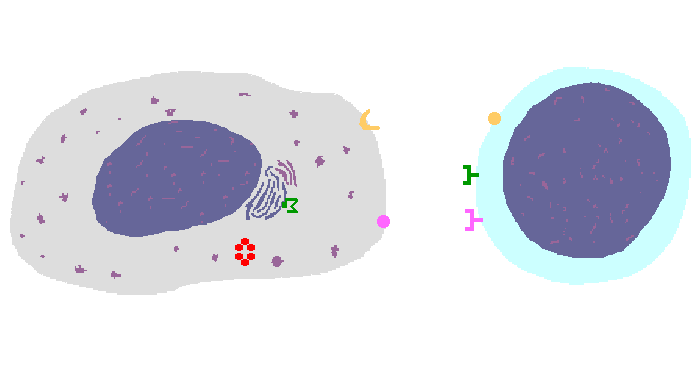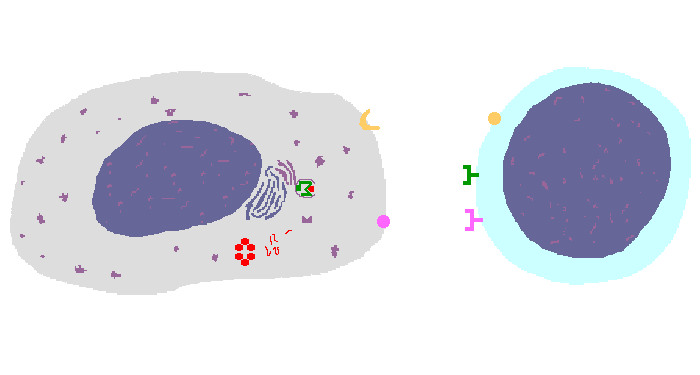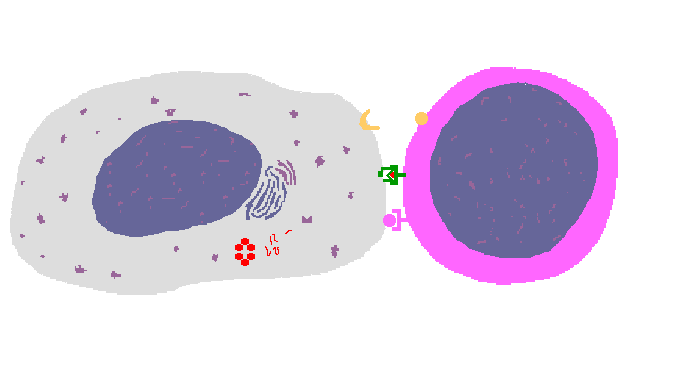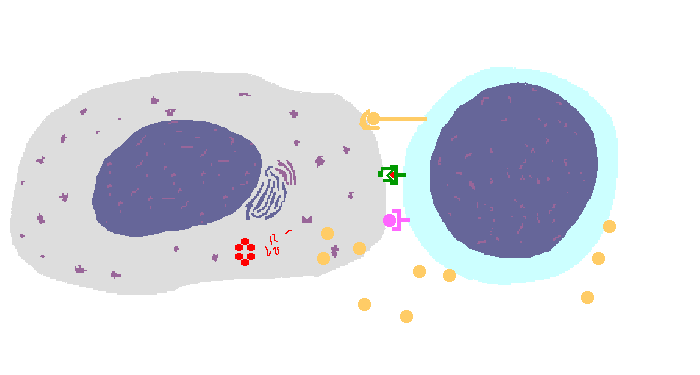
The interaction of a macrophage (an antigen presenting cell) on the left with a CD8 cell on the right is shown above. The key components of this interaction are labelled below. The intracellular microbe living within the macrophage produces antigenic protein that is unfolded, ubiquinated, and digested in a proteasome into antigenic peptides. The MHC I molecule is manufactured in the endoplasmic reticulum (ER), has a beta-2 microglobulin molecule added, and is packaged in the Golgi apparatus. The antigenic peptide fills the binding site of the MHC when fusion with the phagosome carrying microbial peptide fragements occurs. The MHC molecule is transported and displayed on the macrophage surface. The CD8 lymphocyte then interacts with the macrophage, using its T cell receptor (TCR) to recognize the MHC displaying a peptide specific for that TCR. The TCR complex on the CD8 cell consists of variable alpha and beta chains of TCR that recognize the antigen, along with a gamma chain and a CD3 protein that signal the cell when contact with a specific MHC carrying antigen occurs. Adhesion and signal transduction are further aided by a protein specific to the T cell surface (CD8 in this case). Adhesion is also mediated by integrins expressed by the CD8 cell that contact macrophage ligand. This recognition is aided by a costimulator (B7) on the macrophage surface that interacts with CD28 on the T cell surface. This "turns on" the CD8 cell which then uses a CD40 ligand to stimulate the macrophage to increase cytokine production that further activates the CD8 cell to become a cytotoxic cell and to enhance CD8 cytokine production. This mechanism helps to keep immune responses highly specific for a microbe and produce specific antibody against the microbe. This interaction can increase the T cell response. |



 |